Welcome

Welcome to the first issue of SmartFactory Rx ALERTS, a quarterly newsletter for pharma manufacturers looking to improve productivity and quality through innovative improvements in manufacturing practices. ALERTS provides valuable technology and innovation updates, case studies, and an opportunity for you to engage with our team.
Joe Caputo, VP & General Manager, Automation Products Group, Applied Materials
We welcome your feedback and ideas for future issues and look forward to working with you to achieve revolutionary improvements in manufacturing productivity and quality.
Semiconductor to Pharma — Why Pharma?
For over 30 years, Applied Materials has helped semiconductor manufacturers optimize quality, efficiency, and productivity. Our technology and services have enabled the industry to move from semi-auto to full automation and drive business results. Today, 100% of the fully automated wafer factories in the world use Applied Materials’ software automation products.
New Opportunities for Pharma Manufacturing
|
Applied engaged the Boston Consulting Group (BCG) to do an in depth study to determine what other industries have challenges similar to semiconductor, where software automation can be applied to improve:
- Quality
- Yield
- Throughput
- Downtime
- Asset Utilization
|

|
| Fig 1: Manufacturing Efficiency Industry Comparison between Semiconductor and Pharma. |
|
The results of the study, which included expert interviews and deep analysis of over 80 manufacturing industries, identified pharma manufacturing (encompassing both small and large molecule APIs) as a key industry motivated to pursue the next generation manufacturing software solutions.
We have focused on three core areas we believe are critical to the pharma manufacturing industry’s evolution:
- Analytics & Control (real-time monitoring & fault detection, statistical process control (SPC), and advanced process control (APC))
- Operations Productivity (plant and supply chain planning & scheduling)
- Advanced Maintenance (mobile CMMS and predictive & prescriptive maintenance)
World-Class Software and Pharma Expertise
Applied has formed a world-class pharma team consisting of pharma and semiconductor experts who are supported by a 500-person Automation Products Group. In addition, we have organized an external advisory board comprised of pharma industry executives to help navigate our entry into the pharma manufacturing software market. Our advisors come from the FDA, pharma manufacturing, advanced analytics & multivariate analysis, plant & supply chain optimization, and advanced process control, all with pharma orientation and experience.
Applied provides pharma manufacturers an opportunity to take advantage of the lessons learned over decades of experience in the semiconductor industry, to quickly accelerate pharma manufacturers through the evolution of manufacturing. While there are many similarities between semiconductor and pharma, there are also many differences. Applied is eager to engage with forward thinking pharma manufacturers to embark on the journey together to unprecedented levels of productivity and quality.
|
Applied participated in publishing an article in American Pharmaceutical Review that examined the similarities and differences between these industries and how to use Silicon Valley technology in pharma manufacturing.
|

|
We welcome your feedback and thoughts on this and Industry 4.0 ideas. Read article.
What is the Applied SmartFactory® Rx?
|
Applied SmartFactory Rx is a suite of advanced manufacturing software for pharma manufacturers, refined through 30 years of leadership in semiconductor manufacturing.
|
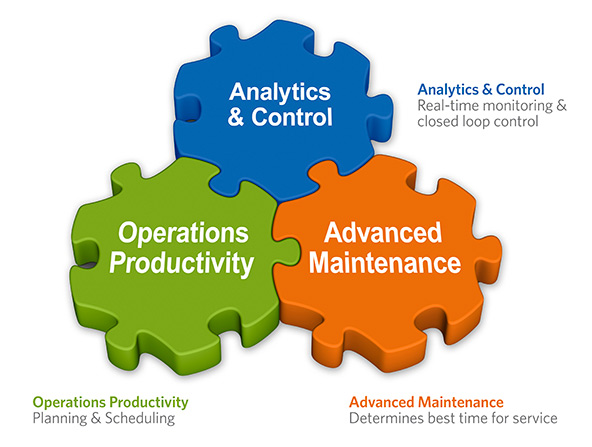
| Fig 2: The SmartFactory Rx suite consists of three integrated components, enabling Pharma users to drive manufacturing efficiency improvement. |
The SmartFactory Rx suite consists of:
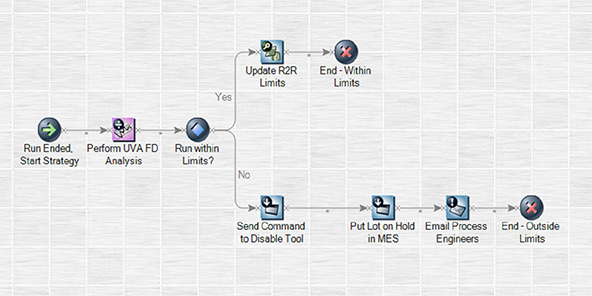
Analytics & Control (A&C) – includes a powerful strategy engine for real-time monitoring, control, and optimization of unit operations. A&C was developed for users with no need for software development experience or training. Strategies can be easily implemented using visual programmatic blocks as shown in the figure below. Learn More
|
|
| Fig 3: Example A&C fault detection strategy shown in the strategy editor UI. |
|
Advanced Maintenance (AM) – implements predictive and prescriptive maintenance strategies. Working cooperatively with A&C to characterize operational behavior patterns, AM predicts and diagnoses anomalies before the actual failure occurs and prescribes maintenance actions. AM is a fully functional CMMS with mobile features, which enables engineers and technicians to work close to the equipment, reducing error and increasing accurate documentation. Learn more
Operations Productivity (OP) – creates efficient and optimal production schedules using rule-based heuristics to maximize productivity and OEE. OP can be used at the plant or supply chain scale for planning and scheduling. Learn More
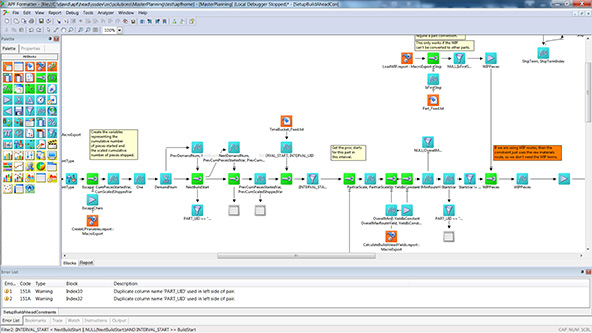 |
| Fig 4: Example dispatching rule shown in the rule formatter UI. |
|
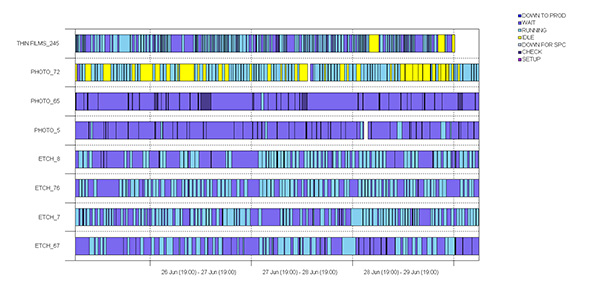 |
| Fig 5: Example Gantt chart of (8) pieces of equipment with the states shown by the colors displayed in the legend, over time (~ 4 days). |
|
Upcoming Industry Events
IFPAC - Puerto Rico | Carolina, Puerto Rico | June 13-14, 2017 | http://ifpacsummit.org/
Don’t miss the opportunity to join Ashley Howard, Applied Materials, Author and Speaker, to discuss Using an Industry 4.0 Approach for Predictive Maintenance
Session: Wed June 14 at 1:30 p.m. – 5:15 p.m. | Location: Salon del Mar A | Track: PAT & Control
Join Lucas Vann, NC State BTEC, author and speaker, to discuss Implementation of Advanced Process Control in Biopharma from Development to Manufacturing. In this presentation Lucas demonstrates how data analytics and PAT (including NIR and soft sensors) were used to increase process understanding around failures due to raw material variability. Integrating multiple data types was required and was accomplished using analytics & control, which also enabled the design of advanced control strategies to achieve greater consistency in product titer and yield from batch to batch.
Session: Wed June 14 8:30 a.m. – 12:00 p.m.| Location: Salon del Mar A | Track: PAT & Control
Contact Us
If you have content suggestions or would like share your software success stories for future issues of SmartFactory Rx ALERTS please send your ideas to SmartFactory_RX@amat.com
|